This is the best summary I could come up with:
“The use of the word ‘cure’ in relation to sickle cell disease or thalassemia has, up until now, been incompatible,” she said in a statement, calling the MHRA’s approval of gene therapy “a positive moment in history.”
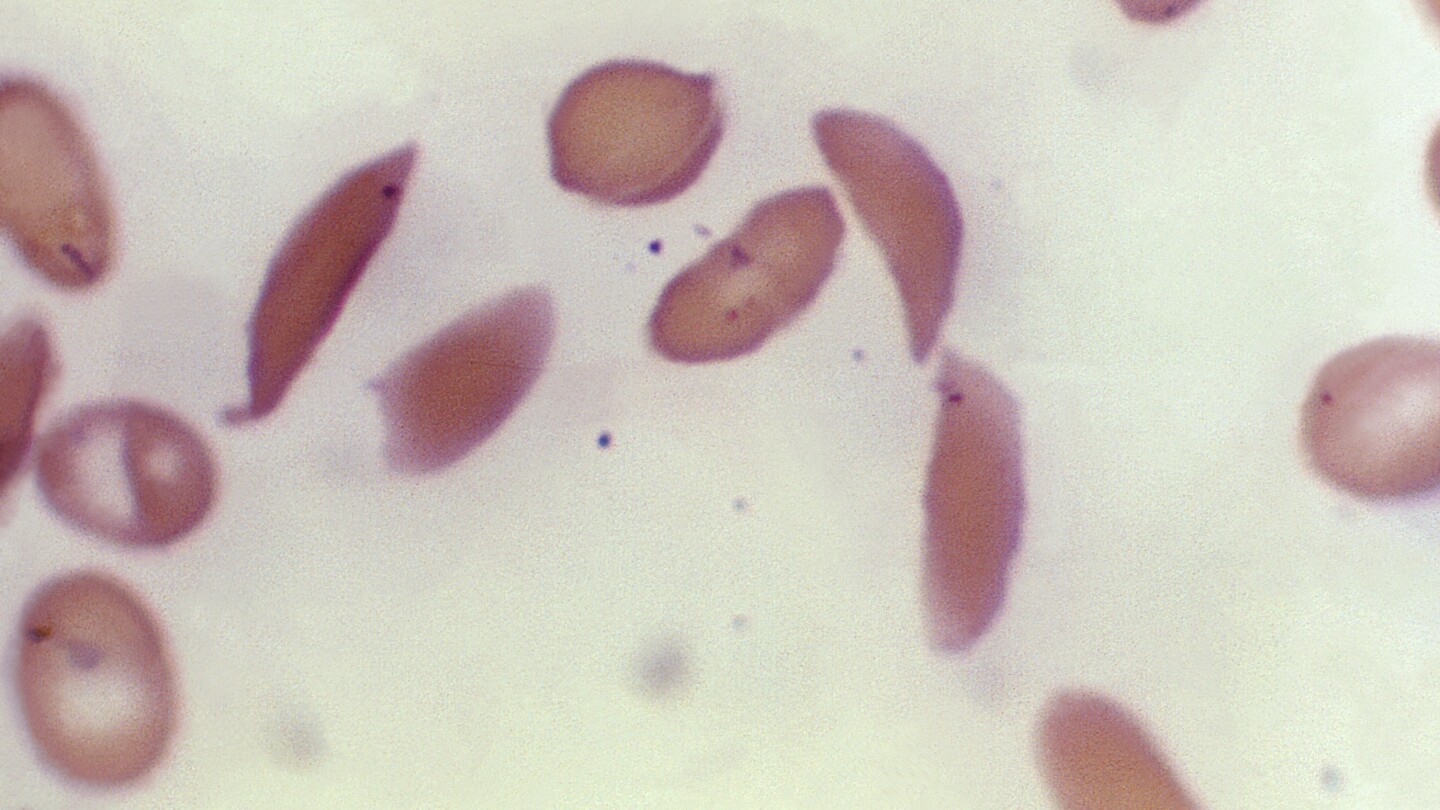
The new medicine, Casgevy, works by targeting the problematic gene in a patient’s bone marrow stem cells so that the body can make properly functioning hemoglobin.
Britain’s regulator said its decision to authorize the gene therapy for sickle cell disease was based on a study done on 29 patients, of whom 28 reported having no severe pain problems for at least one year after being treated.
Gene therapy treatments can cost millions of dollars and experts have previously raised concerns that they could remain out of reach for the people who would benefit most.
Vertex Pharmaceuticals said it had not yet established a price for the treatment in Britain and was working with health authorities “to secure reimbursement and access for eligible patients as quickly as possible.”
Casgevy is currently being reviewed by the U.S. Food and Drug Administration; the agency is expected to make a decision early next month, before considering another sickle cell gene therapy.
The original article contains 774 words, the summary contains 194 words. Saved 75%. I’m a bot and I’m open source!
I’ve been waiting for this ever since I learned about CRISPR! Knowing that sickle cell anemia is the result of a single point mutation, I always figured it would be one of the first diseases cured by gene therapy.