The Food and Drug Administration says 561 deaths have been reported in connection to recalled Philips devices to treat obstructive sleep apnea and other breathing disorders.
The FDA said that since April 2021 it has received more than 116,000 medical device reports of foam breaking down in Philips CPAP (continuous positive airway pressure) machines and BiPAP sleep therapy devices. That includes 561 reports of death, the agency said Wednesday.
The Dutch medical device maker has recalled millions of the breathing machines amid reports they were blowing gas and pieces of foam into the airways of those using the devices.
The grim tally comes days after Philips said it would stop selling the machines in the U.S. in a settlement with the FDA and the Justice Department expected to cost roughly $400 million, the company disclosed in a regulatory filing.


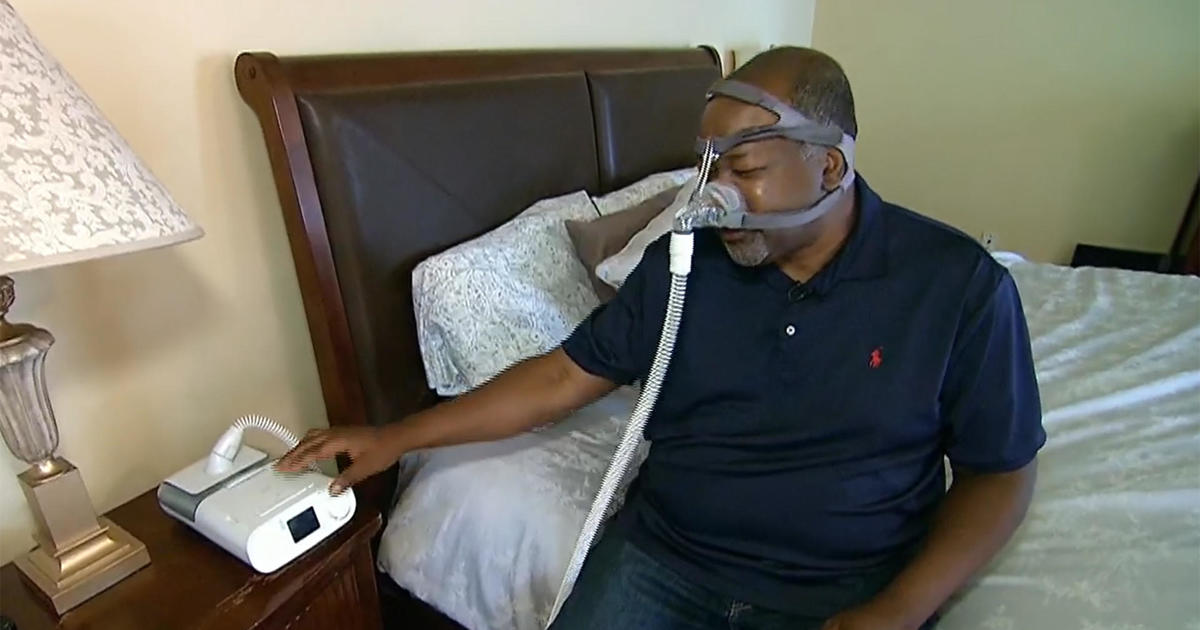
I had a Philips Dreamstation that was recalled. I’m not sure exactly what happened but I started having an issue where I would feel extreme euphoria at random times throughout the day when I inhaled. It only happened when I used the Dreamstation. If I used my travel CPAP or my replacement Airsense, I had no problems.
I saw my PCP about it and we never figured it out. And again, it stopped when I switched devices.
I don’t know what gases were being released but there’s got to be a correlation.
The reason for the recall is due to the sound insulation material breaking down and being breathed in.
This certainly should not cause euphoria :?
Dunno doesn’t sound too bad to me
Not a bug, it’s an undocumented feature.